

Welcome! Click here to login or here to register.
| |
|||||||||||||||||||
|
|||||||||||||||||||
MMR: MutSalpha binds mismatch in heteroduplex DNA with nick 3' to mismatch
[Homo sapiens]
| GO molecular function: 0030983 |
| Mismatch recognition is performed by the MutS protein. In mammals, there are five homologs (MSH2-6) of MutS, with MSH2, MSH3 and MSH6 having a role in MMR. All mismatches and small indels are recognized by MutSα heterodimer (composed of MSH2 and MSH6), while longer indels are recognized by MutSβ (heterodimer of MSH2 and MSH3). First, MutS searches for the mismatch and binds to it in the nucleotide-free or ADP-bound state. The binding of MutS to DNA induces bending of both homo- and heteroduplex DNA. When MutS encounters the mismatch, a bend is turned into a sharp kink. This triggers a conformational change in the protein and the exchange of ADP to ATP. MutS-ATP complex remains stably bound to DNA, but its affinity to the mismatch is reduced. At the same time, the ATP hydrolysis activity of MutS is inhibited. Lastly, the MutS-ATP complex leaves the mismatch and moves along DNA as a sliding clamp to activate downstream repair events near the strand discrimination signal. |
This is the reaction from:
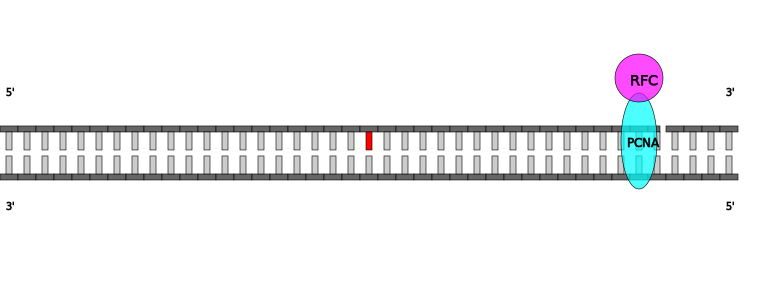 | Heteroduplex DNA with nick 3' to mismatch in Homo sapiens |
| to | |
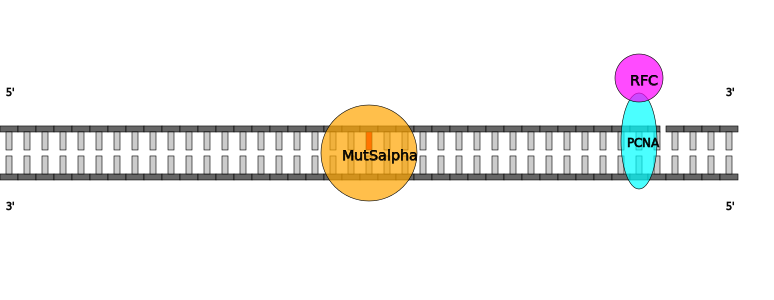 | Mismatch in heteroduplex DNA with nick 3' to mismatch bound by MutSalpha in Homo sapiens |
Involved proteins:
References:
| Authors | Title | Journal |
| Antony E, Hingorani MM | Asymmetric ATP binding and hydrolysis activity of the Thermus aquaticus MutS dimer is key to modulation of its interactions with mismatched DNA. |
|
| Jacobs-Palmer E, Hingorani MM | The effects of nucleotides on MutS-DNA binding kinetics clarify the role of MutS ATPase activity in mismatch repair. |
|
| Lamers MH, Georgijevic D, Lebbink JH, Winterwerp HH, Agianian B, de Wind N, Sixma TK | ATP increases the affinity between MutS ATPase domains. Implications for ATP hydrolysis and conformational changes. |
|
| Tessmer I, Yang Y, Zhai J, Du C, Hsieh P, Hingorani MM, Erie DA | Mechanism of MutS searching for DNA mismatches and signaling repair. |
|
| Wang H, Yang Y, Schofield MJ, Du C, Fridman Y, Lee SD, Larson ED, Drummond JT, Alani E, Hsieh P, Erie DA | DNA bending and unbending by MutS govern mismatch recognition and specificity. |
|
Keywords:
Add your own comment!
There is no comment yet.
|
|

|

|